CDSCO Import License for Medical Devices: How to get MD 15?
Detailed information on CDSCO Import license (MD-15) for the import of medical devices
MD 15 is a CDSCO registration which is needed for import of medical devices into India by an importer.
In India, medical devices are regulated as per Drug & Cosmetic Act 1940, Medical Device Rules 2017. CDSCO (Central Drug Standard Control Organization) is the regulatory authority for the import of medical devices in India.Regarding import of medical devices, an Import license application (Form MD 14) is submitted and registration is granted in Form MD 15.
CDSCO Classification of Medical Device
The CDSCO has classified medical devices into four classes as per the risk associated with their use viz., Class A, B, C, D
Class A devices- these are the lowest-risk devices eg: Cutting and Dissecting Surgical instruments, Prosthetic Ear, Prosthetic Limb, Dry powder inhaler, Steam inhaler, Face Masks etc.
Class B devices- These are low to moderate-risk devices eg: Oximeter, Oxygen Concentrator, Thoracic suction pump, Spirometer, Surgical drape, Skin Stapler, Cardiac monitor, Pacemaker lead adaptor etc.
Class C devices- These are moderate to High-risk devices eg: Implantable staple, Anesthesia machine, High-frequency ventilator etc.
Class D devices- These are the highest-risk devices eg: Endomyocardial biopsy device, Replacement heart valve etc.
Who can apply for CDSCO Import License MD 14?
- Importers and Wholesalers: Obtaining an MD 15 License to Import Medical Devices in India
If you are an importer or wholesaler dealing with medical or in-vitro diagnostic devices in India, it is mandatory to acquire an MD 15 License before importing such devices. This license, granted by the CDSCO, ensures that imported medical devices meet safety and quality standards required for distribution in India.
Once you secure an MD 15 License, you are authorized to import medical devices and in-vitro diagnostic devices for sale under either your trade name or that of the manufacturer. MD 15 includes the name of Authorised agent/Importer, Manufacturer Details and Device Details
- Foreign Manufacturers: Obtaining an MD-15 License to Sell Medical Devices in India
As a foreign manufacturer, you need to obtain an MD 15 License from the CDSCO to sell your medical devices in the Indian market. To apply, you must appoint an authorized agent in India who will manage communications with the CDSCO and handle the application process.
With an MD-15 License, your authorized agent can distribute and sell your medical devices in India. The authorized agent must have MD 42, form 20B or 21B for the trading purpose.
What is the deadline for obtaining an MD 15 license for importing medical devices into India as per CDSCO registration?
The deadline for all medical device importers to obtain an MD 15 License from the CDSCO was October 1, 2023. After this date, importing medical devices into India will not be permitted without a valid MD 15 License.
Documents required to get CDSCO import license
- Power of Attorney authenticated in India either by a Magistrate of First Class or by the Indian Embassy in the country of origin or by an equivalent authority through apostille along with undertaking from the authorized agent.
- Self-attested copy of valid manufacturing license.
- Duly apostilled/notarized copy of Free Sale Certificate, Marketing Authorization of the product from National Regulatory Authority of country of origin.
- Copy of latest inspection or audit report carried out by Notified bodies or National Regulatory Authority or Competent Authority within last 3 years.
- Notarized copy of ISO 13485 Certificate of Actual Manufacturer
- Notarized Full Quality Assurance Certificate/CE type examination Certificate/CE product quality assurance.
- Notarized CE design Certificate
- Notarized Declaration of conformity
- Notarized copy of overseas manufacturing site or establishment or plant registration, by whatever name called, in the country of origin issued by the competent authority
- Constitution details of domestic authorized agent
- Plant Master file from the Manufacturer
- Device Master file from the Manufacturer.
The device master file should include the following key points for a comprehensive documentation:
- Executive Summary of the Device
- Reference to predicate or previous generations of the device
- Label & Instructions for Use
- Device Design and Manufacturing process with flow chart
- Essential Principles Checklist
- Risk analysis and control summary
- Design Verification and validation of the medical device
- Biocompatibility validation data
- Medicinal substances data if the device contains Drug
- Biological Safety (TSE/BSE)
- Sterilization Validation data, if the device is supplied in a sterile state
- Software verification and validation, if the device incorporates any software
- Animal studies Preclinical data
- Stability validation data
- Clinical evidence
- Post Marketing Surveillance data
- Batch Release Certificates or Certificate of Analysis of finished product for minimum 3 consecutive batches.

CDSCO Import License Application Process
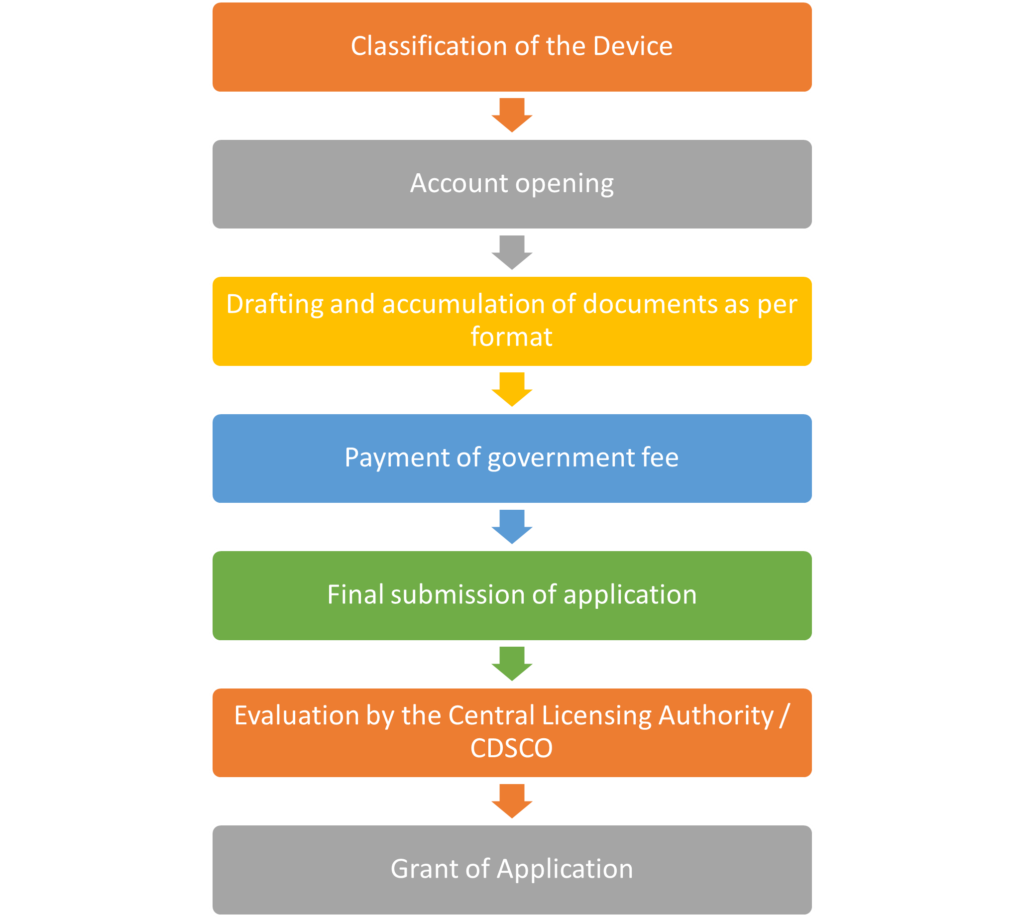
- Classification of the Device:
This step involves assigning regulatory class based on its intended use and potential risk to the patient or user. - Account Opening:
The manufacturer or applicant needs to create an account with the CDSCO online portal, which will be used to submit the application and monitor its status. - Drafting and Accumulation of Documents:
In this stage, the required documents such as technical data, safety and performance reports, and compliance certifications are prepared according to the regulatory format. These documents form the basis of the application. - Payment of Government Fee:
The applicant is required to pay a regulatory or government fee, which is a necessary part of the application process. The amount varies depending on the class of the device. - Final Submission of Application:
After compiling all the necessary documents and completing the fee payment, the final application is submitted to the authority for consideration. - Evaluation by the Central Licensing Authority / CDSCO:
The submitted application is thoroughly reviewed and evaluated by the regulatory authority (Central Licensing Authority or CDSCO). They assess the device’s compliance with regulatory standards and requirements. - Grant of MD 15:
If the application satisfies all regulatory requirements and the device is deemed safe and effective, the authority grants approval, allowing the device to be legally marketed or used.
CDSCO Import License MD-15 Government Fee
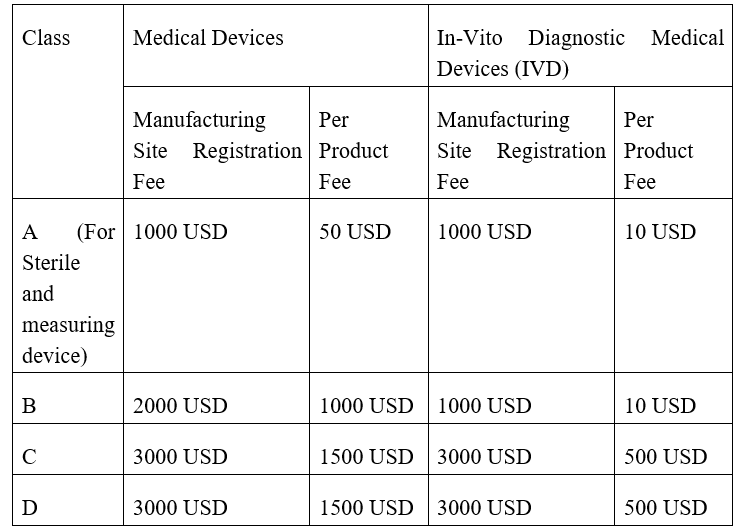
In the case of Class A non-sterile non-measuring medical devices there is no government fee.
Timeline for Import License MD 15
After the submission of the application, the import license (MD 15) is granted within 120 to 180 working days.
Frequently Asked Questions
CDSCO Import License for Medical Devices: How to get MD 15 ?
A medical device import authorization is issued by the Central Drugs Standard Control Organization (CDSCO) in Form MD 15 that permits the entry of medical devices into India.
Form MD-14 is the CDSCO application form required for importing medical devices into India. When a foreign producer wants to export medical devices to India, this application must be submitted through an authorized Indian agent or distributor, who must hold a valid wholesale license (Form MD-42) for selling medical devices in India.
The MD-15 license, granted by the CDSCO, is necessary for the import of medical devices and in-vitro diagnostic devices into India. This license is a compulsory requirement for bringing any medical device into the country.
Applications for a Medical Device Import license should be made using Form MD-14, while the authorization is issued in the form of MD-15.
Individuals or entities looking to import medical devices into India for commercial purposes must obtain a CDSCO medical device import license.
To apply for a medical device import license, you must submit the required documents and information through the CDSCO’s online portal along with the prescribed fee.
The time required to process a medical device import license usually ranges from 3 to 6 months. The duration depends on the completeness of the application, the complexity of the device, and the current workload of the regulatory bodies.
Yes, importers must adhere to the Medical Device Rules of 2017, which include regulations on device classification, labeling requirements, quality management systems, and post-market surveillance.
No, importing medical devices into India without the appropriate import license is not permitted. If you do so, your consignment will get stuck during custom clearance
Medical Device Registration Services CDSCO Import License MD 15
Get expert support for CDSCO medical device registration. Contact us for a free consultation call today!
Contact Us
Get free call today !
![]() +91 9891232884
+91 9891232884
![]() info@cdscoregistration.com
info@cdscoregistration.com
