CDSCO Medical Device manufacturing license: How to get MD5 license ?
MD-5: CDSCO Medical device manufacturing license of Class A and B Medical Devices
At cdscoregistration.com, we assist individuals and businesses in obtaining the necessary Registration Certificate to legally sell, stock, exhibit, or distribute medical devices, including in vitro diagnostic medical devices, within India.
Manufacturers of Class A (excluding non-sterile and non-measuring devices) and Class B medical devices are required to obtain an MD-5 certificate for manufacturing or distribution in India. The process, governed by the Medical Device Rules (MDR), 2017, ensures that all medical devices meet high standards of safety, efficacy, and quality before reaching the market. MD-03 is the application form which is submitted to the state licensing authority and MD-05 is granted as the registration certificate for the manufacturing purpose.
Whether you’re a local manufacturer or a global company looking to enter the Indian market, compliance with these regulations is essential to legally produce and distribute medical devices
Examples of Class A sterile & Measuring Devices: Amino acid analyser, Lipid profile analyser, Breath-alcohol test system, Osmometer, Blood cell count analyser, Sterile Dental dressing forceps, Templates, Periostic elevator etc.
Examples of Class B Devices: Spirometer, Oxygen Concentrator, Blood Pressure Monitoring Device Digital Thermometer etc.
.
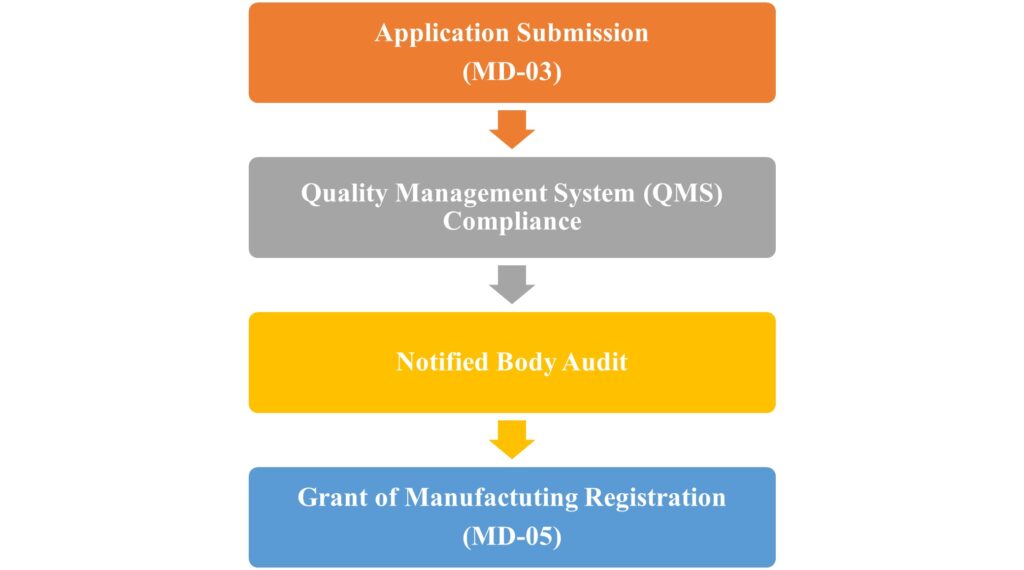
- Application Submission
Manufacturers intending to produce Class A (sterile/measuring) or Class B medical devices must apply for a manufacturing license through the Ministry of Health and Family Welfare’s online portal. The application must include all required documents as per the Indian Medical Device Rules 2017 - Quality Management System (QMS) Compliance
A key part of the MD-5 process is ensuring that your manufacturing facility meets the Quality Management System (QMS) standards. Class A devices do not require an on-site audit prior to receiving the license, but Class B devices require an audit by a registered Notified Body. - Notified Body Audit
For Class B devices, a comprehensive audit of the manufacturing site will be conducted by a Notified Body within 90 days of application. This ensures that your facilities conform to the applicable standards. The audit report is submitted to the State Licensing Authority, which then reviews the findings. - License Issuance
If the application and audit report meet all regulatory requirements, the State Licensing Authority will issue the MD-5 license within 20 days of receiving the audit report. For Class A devices, the process is even faster, with no pre-license audit required. The license for Class A devices will be issued within 45 days of application.
Goverment fee
The government fee for MD-03 is ₹5,000 for registering the manufacturing site, plus ₹500 for each device you want to register.
Requirements for Application: CDSCO Medical Device Manufacturing license
When applying for the MD5 cdsco medical device manufacturing License, your submission must include the following:
- Constitution Details
- Site or Plant Master File
- Device Master File
- Test License
- Compliance Undertaking
- Additional Documents: Label, Product broachers & IFU
- Performance Evaluation Report (for In-Vitro Diagnostic Devices)
Approval Process:
Why Choose Us for CDSCO Medical Device Manufacturing license: MD-5 Registration?
At cdscoregistration.com, we offer comprehensive support through every step of the MD-5 licensing process. Our team, which includes regulatory experts, chemists, and engineers, ensures that your application is meticulously prepared, saving you time and effort.
We provide the following services:
- Guidance on QMS compliance to meet the Fifth Schedule requirements.
- Dossier preparation and Application submission
- Audit coordination with a registered Notified Body for Class B devices.
- Ongoing support for maintaining compliance and renewing your license as needed.
Simplify Your Medical Device Manufacturing Registration
The MD-5 registration process may seem complex, but with cdscoregistration.com as your partner, you can focus on innovation while we handle the regulatory work. Whether you are manufacturing Class A or Class B medical devices, we ensure you meet all legal requirements swiftly and efficiently.
Let us help you bring safe, compliant medical devices to market—contact us today to get started on your MD-5 registration!
Frequently Asked Questions
CDSCO Medical Device manufacturing license: How to get MD5 license ?
The MD-5 registration is a certificate required for manufacturers of Class A (excluding non-sterile and non-measuring devices) and Class B medical devices to legally manufacture or distribute their products in India, as per the Medical Device Rules (MDR), 2017.
The MD-03 application form is submitted to the State Licensing Authority to apply for a license to manufacture or import medical devices. Upon approval, the MD-5 certificate is granted for manufacturing purposes.
- Examples of Class A (Sterile/Measuring) Devices:
- Amino acid analyzers
- Lipid profile analyzers
- Breath-alcohol test systems
- Osmometers
- Blood cell count analyzers
- Sterile dental dressing forceps
- Examples of Class B Devices:
- Spirometers
- Oxygen concentrators
- Blood pressure monitoring devices
- Digital thermometers
- Application Submission: Submit the MD-03 application via the Ministry of Health and Family Welfare's online portal, including all required documents.
- Quality Management System (QMS) Compliance: Ensure that the manufacturing facility meets QMS standards. Class A devices do not require an audit before licensing, while Class B devices do.
- Notified Body Audit: For Class B devices, an audit will be conducted within 90 days of application submission.
- License Issuance: If all requirements are met, the MD-5 license will be issued within 20 days for Class B devices and 45 days for Class A devices.
The government fee for submitting the MD-03 application is ₹5,000 for registering the manufacturing site, plus an additional ₹500 for each device being registered.
The following documents are required:
- Constitution details of the domestic manufacturer or authorized agent.
- Site or plant master file.
- Device master file.
- Test license (for domestic manufacturers).
- Compliance undertaking stating adherence to the Fifth Schedule.
- Additional documents such as labels, product brochures, and Instructions for Use (IFU).
- Performance evaluation report (for in vitro diagnostic devices).
cdscoregistration.com offers comprehensive support throughout the MD-5 licensing process, including:
- Guidance on QMS compliance.
- Dossier preparation and application submission.
- Audit coordination for Class B devices.
- Ongoing support for maintaining compliance and renewing licenses.
Compliance with MD-5 is essential for manufacturers to ensure that their medical devices meet safety, efficacy, and quality standards, allowing them to legally produce and distribute their products in the Indian market.
If you need assistance with the MD-5 registration process, you can contact us at cdscoregistration.com for expert guidance and support to simplify your medical device manufacturing registration.
The overall timeline for grant of MD-05 takes 4 to 6 months.
The Notified Body is responsible for conducting a comprehensive audit of the manufacturing site for Class B devices. Their assessment ensures that the facility complies with applicable standards and regulations. The audit report submitted by the Notified Body is critical for the State Licensing Authority’s decision-making.
The QMS is crucial for ensuring that manufacturing processes meet consistent quality standards. It encompasses practices and procedures that manufacturers must adhere to in order to maintain compliance with the Fifth Schedule and ensure the safety and efficacy of medical devices.
Manufacturers should:
- Review and ensure compliance with relevant standards and regulations.
- Maintain thorough documentation of their QMS and manufacturing processes.
- Conduct internal audits to identify and address potential non-conformities.
- Train staff on compliance requirements and audit procedures.
Yes, in vitro diagnostic medical devices require a performance evaluation report to be submitted with the application. This report must demonstrate the device's effectiveness and compliance with relevant standards.
To ensure your application is complete:
- Use a checklist of required documents, including constitutional details, master files, compliance undertakings, and performance evaluation reports.
- Confirm that all documents are properly signed and dated.
- Review the application thoroughly for accuracy and completeness.
Yes, manufacturers must continuously comply with regulatory requirements and maintain the QMS. This includes periodic audits, updating documentation, and renewing licenses as needed. Regular training for staff on compliance is also advisable.
Significant changes to the manufacturing process or device specifications may require re-evaluation and re-submission of documents to the State Licensing Authority. It's essential to consult with us before making changes.
cdscoregistration.com provides ongoing support for maintaining compliance, including:
- Regular updates on regulatory changes.
- Assistance with license renewals and re-evaluations.
- Training programs for staff on compliance and quality management.
- Audit preparation and support to ensure continued adherence to standards.
Medical Device Registration Services MD5 CDSCO Medical Device Manufacturing license
Get expert support for CDSCO medical device registration. Contact us for a free consultation call today!
Contact Us
Get free call today !
![]() +91 9891232884
+91 9891232884
![]() info@cdscoregistration.com
info@cdscoregistration.com
