Medical Device Test Licence in India: Forms MD 16 and MD-17 License
Import of Medical Devices for Testing, Evaluation, and Clinical Investigations
At AccordMed, we assist businesses and individuals in obtaining the necessary licenses for importing medical devices for testing, evaluation, clinical investigations, demonstration, or training purposes. One of the key licenses required for these activities is the MD-17 License, granted by the Central Licensing Authority under the Medical Devices Rules, 2017.
What is the MD-17 License?
To get the MD-17 License we submit the MD-16 Application form i.e., the application to import medical devices for the testing, evaluation, and clinical investigation to the CDSCO. The MD-17 License is a special permit that allows the import of medical devices or in vitro diagnostic devices solely for the purpose of:
- Clinical investigations
- Tests and evaluations
- Demonstrations
- Training purposes
How to Obtain the MD-17 License?
To import medical devices for the purposes mentioned above, an application needs to be submitted to the Central Licensing Authority (CDSCO) in Form MD-16. This form must be accompanied by the prescribed government fee of $100 per device.
Process for Importing Medical Devices for Testing
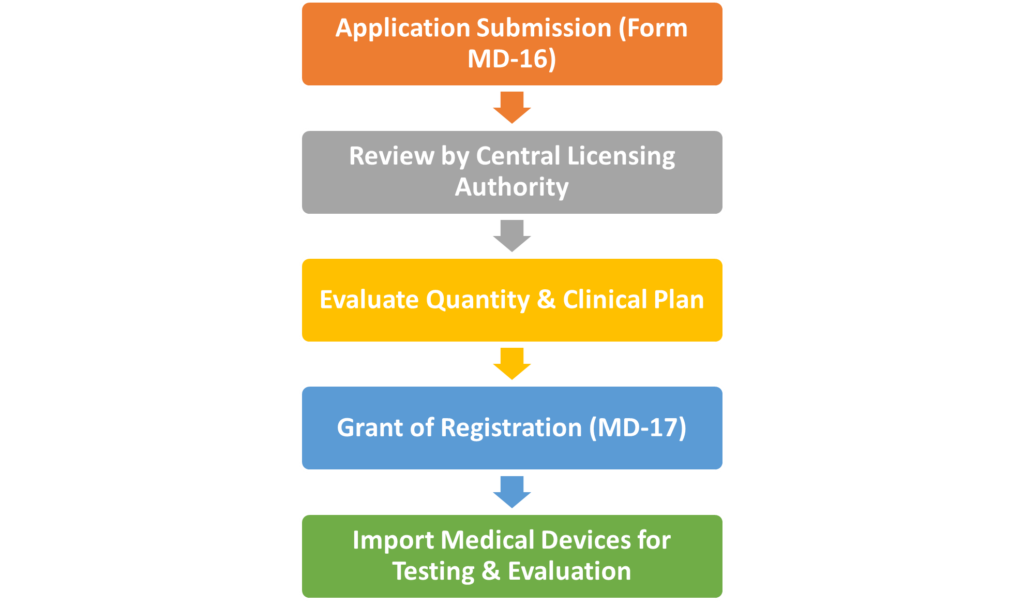
- Application Submission: Applicants must submit Form MD-16, detailing the devices to be imported, the purpose (clinical investigations, testing, etc.), and other required information.
- Review and Assessment: The Central Licensing Authority reviews the application, evaluates the need for the quantity of devices, and checks the submitted clinical investigation plan and relevant documents.
- Granting of License: If all requirements are met, the test license is granted in Form MD-17 within 30 working days from the date of application.
- Usage: The imported devices can only be used for the specific purposes mentioned (clinical investigations, testing, demonstration, or training) and must be used at the locations specified in the license.
Documents needed:
- Brief description of the medical device, including intended use, material of construction, design
- Justification for the quantity proposed to be imported
- Test protocol/Approved clinical investigation plan
- Quality certificates (like QMS, etc.) of the manufacturer
- Labels and Instructions for Use (IFU)
- Digital Signature Certificate (DSC)
Key Points to Note:
- The license is valid for three years.
- The consignment must be accompanied by an invoice or statement showing the name and quantity of the medical device.
- The MD-17 License holder must maintain detailed records of all activities, including information about the manufacturer, quantity imported, and date of import.
- Unused devices may be exported or destroyed, but prior intimation to the Central Licensing Authority is required.
At AccordMed, our team of experts helps streamline the entire process of obtaining the MD-17 License for import, ensuring compliance with regulatory requirements and smooth approval.
Contact us today to get started with your MD-16 License application!
FAQs: Import of Medical Devices for Testing, Evaluation, and Clinical Investigations
What is the MD-17 License?
Who needs to apply for the MD-17 License?
How do I apply for the MD-17 License?
Do I need to maintain records of the imported devices?
Can AccordMed assist with obtaining the MD-17 License?
What documents are required to obtain the MD-17 License?
How long does it take to get the MD-17 License?
How long is the MD-17 License valid?
Can the imported medical devices be used for other purposes?
What should I do with unused medical devices after the testing or investigation?
Import of Medical Devices for Testing, Evaluation, and Clinical Investigations CDSCO MD-17 License
Get expert support for CDSCO medical device registration. Contact us for a free consultation call today!
Contact Us
Get free call today !
![]() +91 9891232884
+91 9891232884
![]() info@cdscoregistration.com
info@cdscoregistration.com
